EBVALLO IN A NUTSHELL
EBVALLO is the first and only on-demand allogeneic T-cell immunotherapy approved for the treatment of relapsed or refractory EBV+ PTLD3
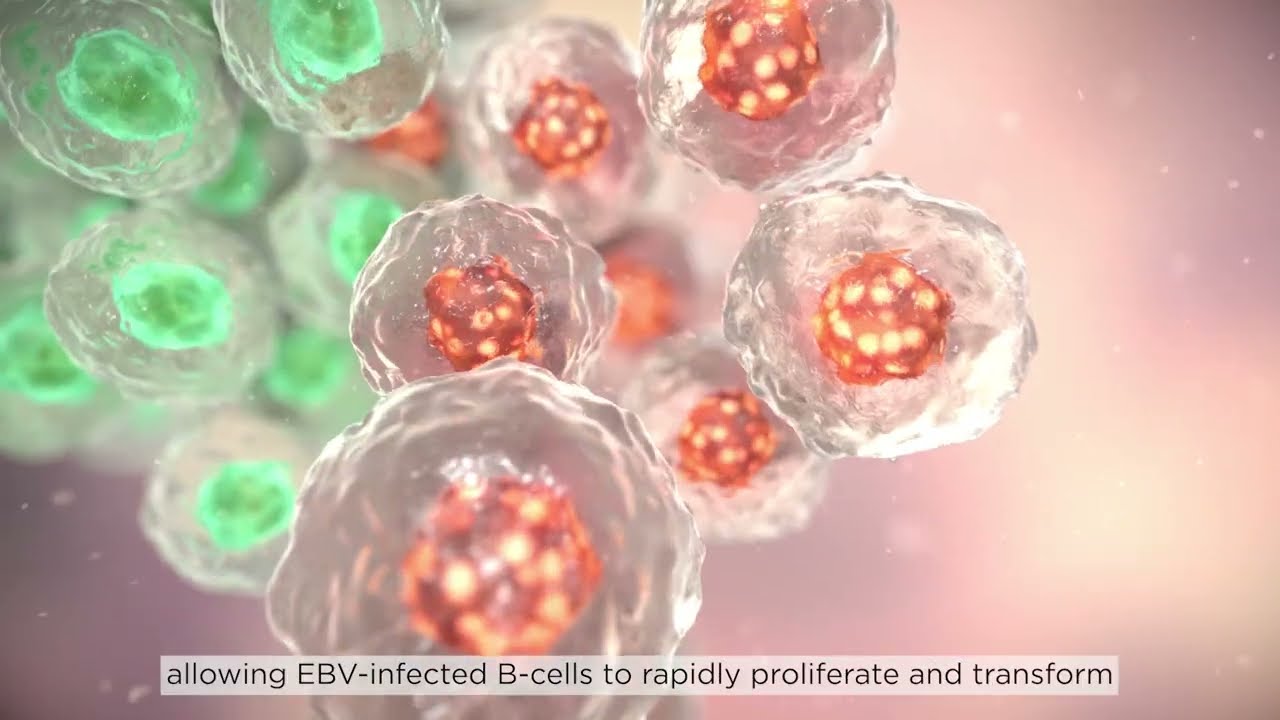
Anti-EBV-specific cytotoxic T-cells
Allogeneic: expanded from third-party donors
Not genetically
modified
Patient HLA
compatible
Stocked in an on-demand biobank
EBVALLO selectively eliminates EBV-transformed tumour cells while sparing healthy cells3
EBV+, Epstein-Barr virus positive; HLA, human leukocyte antigen; PTLD, post-transplant lymphoproliferative disease
MODE OF ACTION
This is a simplified example of the mechanism of action
MANUFACTURING & LOGISTICS
EBVALLO is manufactured from healthy EBV+ donors with diverse HLA profiles to produce expanded CTL lots that are characterised by EBV-specific cytotoxicity and HLA restriction2
EBVALLO is specifically selected for each patient, based on the patient’s disease HLA profile2,3
ClinicalTrials.gov Identifier: NCT03394365
APC, antigen-presenting cell; CTL, cytotoxic T lymphocyte; EBV+, Epstein-Barr virus positive; HLA, human leukocyte antigen; PBMC, peripheral blood mononuclear cell; PTLD, post-transplant lymphoproliferative disease
TREATMENT SCHEDULE & ALGORITHM
Each cycle of EBVALLO lasts approximately 35 days – the number of EBVALLO cycles required depends on patient’s response3
The number of cycles of EBVALLO is determined by response to treatment3
*CR at the end of a cycle followed by PR or other response at any subsequent cycle is considered PD3.
D, day; CR, complete response; IR, indeterminate response; PD, progressive disease; PR, partial response; SD, stable disease.