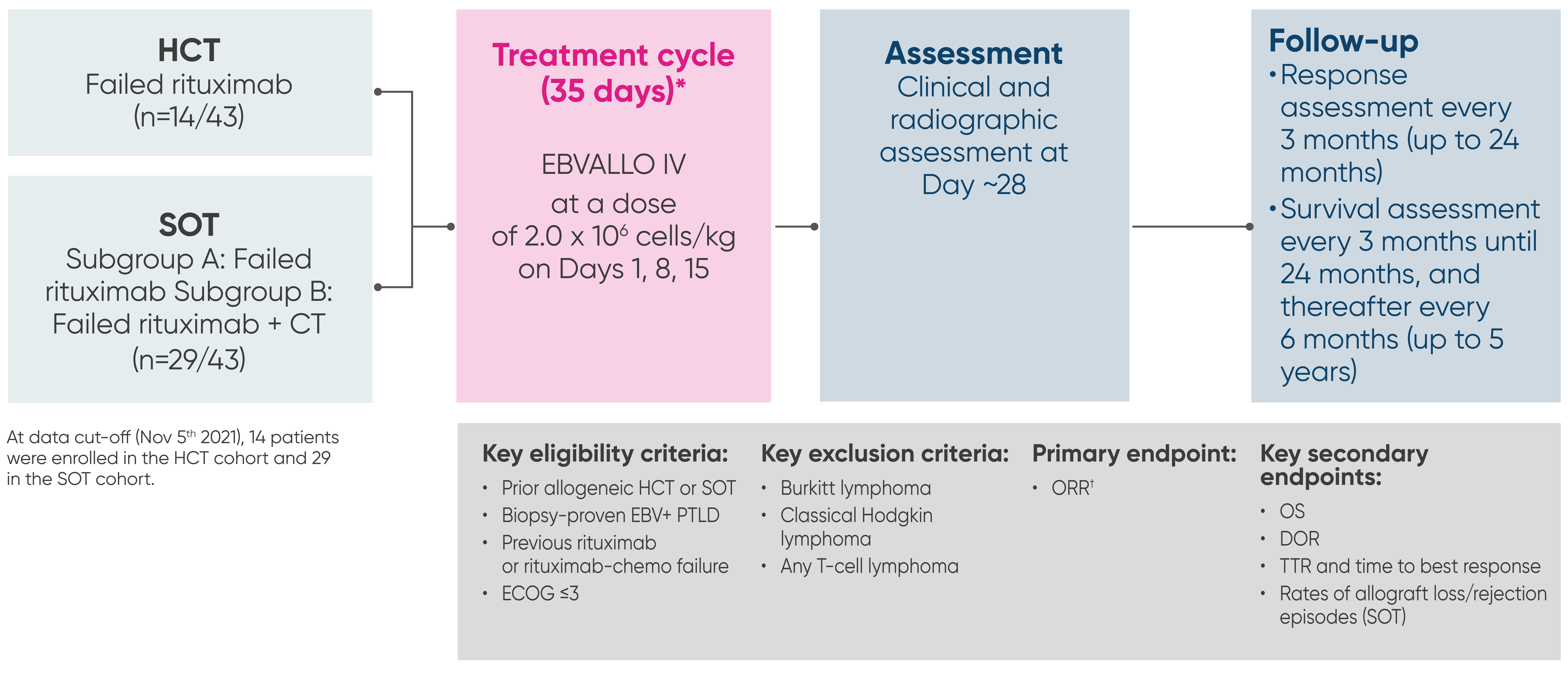
ALLELE STUDY DESIGN
A multicentre, open-label, pivotal study of EBVALLO after failure of rituximab ± CT in patients with EBV+ PTLD following HCT or SOT1
*Treatment ends with any of the following: maximal response achieved, unacceptable toxicity, initiation of non-protocol therapy, failure of up to 4 lots with different HLA restrictions (HCT) or 2 lots with different HLA restrictions (SOT). Please refer to treatment algorithm for more details. †Evaluated by independent review (IORA).
CT, chemotherapy; DOR, duration of response; EBV+, Epstein-Barr virus positive; ECOG, Eastern Cooperative Oncology Group; HCT, haematopoietic cell transplant; HLA, human leukocyte antigens; IV, intravenous; ORR, objective response rate; OS, overall survival; PTLD, post-transplant lymphoproliferative disease; SOT, solid organ transplant; TTR, time to response.
OBJECTIVE RESPONSE RATE (ORR)
In the ALLELE study, 51% of patients responded to EBVALLO with rates consistent across SOT and HCT cohorts1
Data cut-off: November 5th, 2021.
CR, complete response; DOR, duration of response; EBV+,Epstein-Barr virus positive; HCT, haematopoietic cell transplantation; ORR, objective response rate; OS, overall survival; PR, partial response; PTLD, post-transplant lymphoproliferative disease; SOT, solid organ transplantation; TTR, time to response.
BEST RESPONSE RATE
In the ALLELE study, patients responded rapidly and durably to EBVALLO1
*Independent oncologic response adjudication (IORA)-assessed response
Patients not obtaining a partial or complete response may still benefit from switching to a new lot with a different HLA restriction1
DOR, duration of response; EBV+,Epstein-Barr virus positive; HLA, human leukocyte antigen; ORR, objective response rate; OS, overall survival; PTLD, post-transplant lymphoproliferative disease; SOT, solid organ transplantation; TTR, time to response.
OVERALL SURVIVAL (OS)
ALLELE: Patients have a median OS of 18.4 months in the overall population (HCT and SOT)1
1-years OS rate after treatment with EBVALLO1
Responders (n=22)
(95% CI: 58.9–94.7)
Non-responders (n=21)
(95% CI: 14.6–56.1)
Data cut-off: November 5th, 2021.
HCT: Haematopoietic cell transplant; OS: overall survival; SOT: solid organ transplant; CR: complete response; PR: partial response; SD: stable disease; ALLELE Study: A multicentre, open-label, pivotal study of EBVALLO after failure of rituximab ± CT in patients with EBV+ PTLD following HCT or SOT.